Abstract
Background:
Patients with 'accelerated phase' MPNs (MPN-AP, 10-19% blasts) and post-MPN AML (MPN-BP, ≥20% blasts) have a poor prognosis. There are few therapeutic options for this patient group, and their advanced age precludes the use of stem cell transplant (HSCT) in most cases.
Ruxolitinib (RUX) is licensed and approved for use in Myelofibrosis related symptoms and for Polycythaemia Vera resistant/intolerant to hydroxyurea. Azacitidine (AZA) is licensed and approved for treatment of AML and adult patients with high risk MDS not eligible for HSCT. The limited clinical data available suggests that monotherapy of advanced phase MPNs /post-MPN AML with either of these agents is unlikely to produce significant and durable responses. Their use in combination provides an attractive opportunity to fully exploit their differing mechanisms of action.
PHAZAR is a phase Ib dose finding study to establish the maximum tolerated dose and safety of RUX combined with AZA in patients with advanced phase MPNs, including MDS or AML arising from MPN; and to investigate the clinical activity of such combination therapy.
Methods:
A modified two-stage continual reassessment method design with flexible cohort sizes was implemented with a recruitment target of 34 patients. Successive cohorts of 3-5 patients were enrolled at a fixed AZA dose of 75 mg/m2 s/c for 7 days (excluding weekends, on a '5+2+2' schedule) for up to 6 x 28-day cycles in combination with continuous administration of an allocated oral RUX dose (dose levels 0, 1, 2 and 3 = 10, 15, 20 and 25 mg bid respectively).
Toxicities were recorded according to the CTCAE v4.0 and patients evaluated for dose limiting toxicities (DLT) after completion of 1 cycle of treatment. DLTs were defined as grade 3 or 4 non-haematological toxicities occurring during the first cycle and considered related to the trial IMPs. Fever, febrile neutropenia, infections and sepsis were excluded from DLT definition. Key secondary endpoints included best response after cycles 3 and 6, duration of response, PFS and OS. Response assessment was dependent on initial blast % and according to the relevant published criteria: Cheson et al (Blood 2006: 108, p419-25) for MPN-AP and Mascarenhas et al (Leuk Res, 2012: 36, p1500-4) for MPN-BP.
Germline DNA, blood and aspirate and trephine marrow samples were obtained at baseline and every 3 cycles for molecular studies. QoL data measured by the MPN SAF; EQ-5D-5L and EORTC QLQ-C30 questionnaires was obtained at baseline and cycles 1, 2, 4 and 6.
Results:
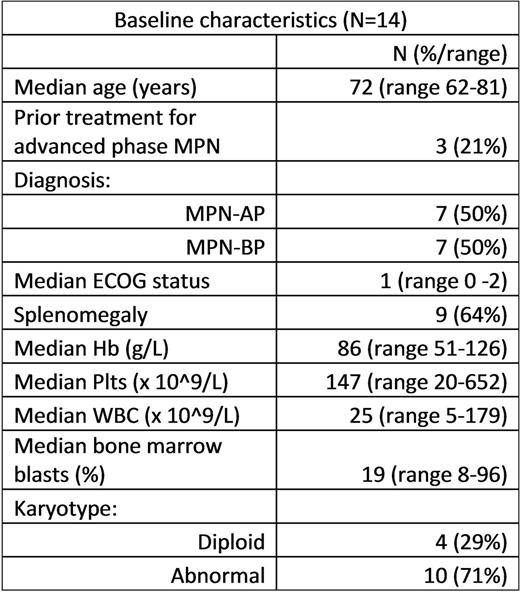
At time of submission, 14 advanced phase MPN patients (11 previously untreated and 3 relapsed/refractory) had been enrolled between 05-Jan-2016 and 30-Jun-2017 (7 MPN-AP; 7 MPN-BP). Baseline characteristics are summarized in Table 1.
Three patients each received 10, 15 and 25 mg of RUX bd, while 4 patients received 20 mg RUX bd. One patient recruited never started trial treatment due to disease progression. The median number of cycles received was 3 (range 0-7). Most adverse events (AEs) reported were grade 1 (47.8%) or grade 2 (24.5%), the most common AEs being constipation, vomiting, anaemia, nausea, abdominal pain, injection site reaction, dyspnoea, fever and hyperkalaemia. Grade 3 or 4 AEs judged at least possibly related to the trial IMPs included anaemia, febrile neutropenia, neutropenia, abdominal pain, hypotension, infections, thrombocytopenia, pneumonitis and syncope. No DLTs occurred. Serious adverse reactions reported included 6 cases of febrile neutropenia, 4 infections and 2 episodes of fever.
Nine patients (64%) remain alive (median length of follow-up: 93 days). Three deaths were considered related to the underlying disease, 1 death to both disease and trial treatment and 1 death related to other cause (sepsis). Six patients remain on treatment and 3 are in follow-up (2 beyond 1 year follow-up). Of the 14 patients recruited, 6 patients were evaluable for disease response at the time of submission. After 3 cycles, 1 patient had complete remission, 2 patients had stable disease and 2 patients had progressive disease. After 6 cycles, 1 patient had acute leukaemia response - partial.
Conclusions:
Initial results suggest that the combination of RUX and AZA is relatively well tolerated in this elderly frail population, with evidence of response seen in some of the evaluable patients thus far (ORR 33%, 2 of 6 patients). Recruitment is ongoing and safety and activity data will be updated at time of presentation.
Drummond: Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Harrison: Gilead: Consultancy, Speakers Bureau; Shire: Speakers Bureau; Celgene: Consultancy; Novartis: Honoraria, Research Funding, Speakers Bureau; CTI: Speakers Bureau. Mead: BMS: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau. Yap: Celgene: Honoraria. Chen: Novartis: Honoraria, Other: Advisory Board. Milojkovic: Incyte: Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Knapper: Novartis: Consultancy, Honoraria, Other: Advisory Board; Celgene: Other: Travel / Conference Support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal